
Gender, Age, Location & Compensation: What Would Make You Participate in a Clinical Trial?
Recent survey of Americans uncovered why people may or may not take part in medical research
Despite a surge in research funding and innovation in digital health, the United States is facing a critical bottleneck in medical progress as too few people are signing up for clinical trials. Our recent nationwide survey of 501 Americans from cities as big as New York, LA, Dallas, and Atlanta to as small as Savannah, Bakersfield, and Biloxi reveals striking findings.
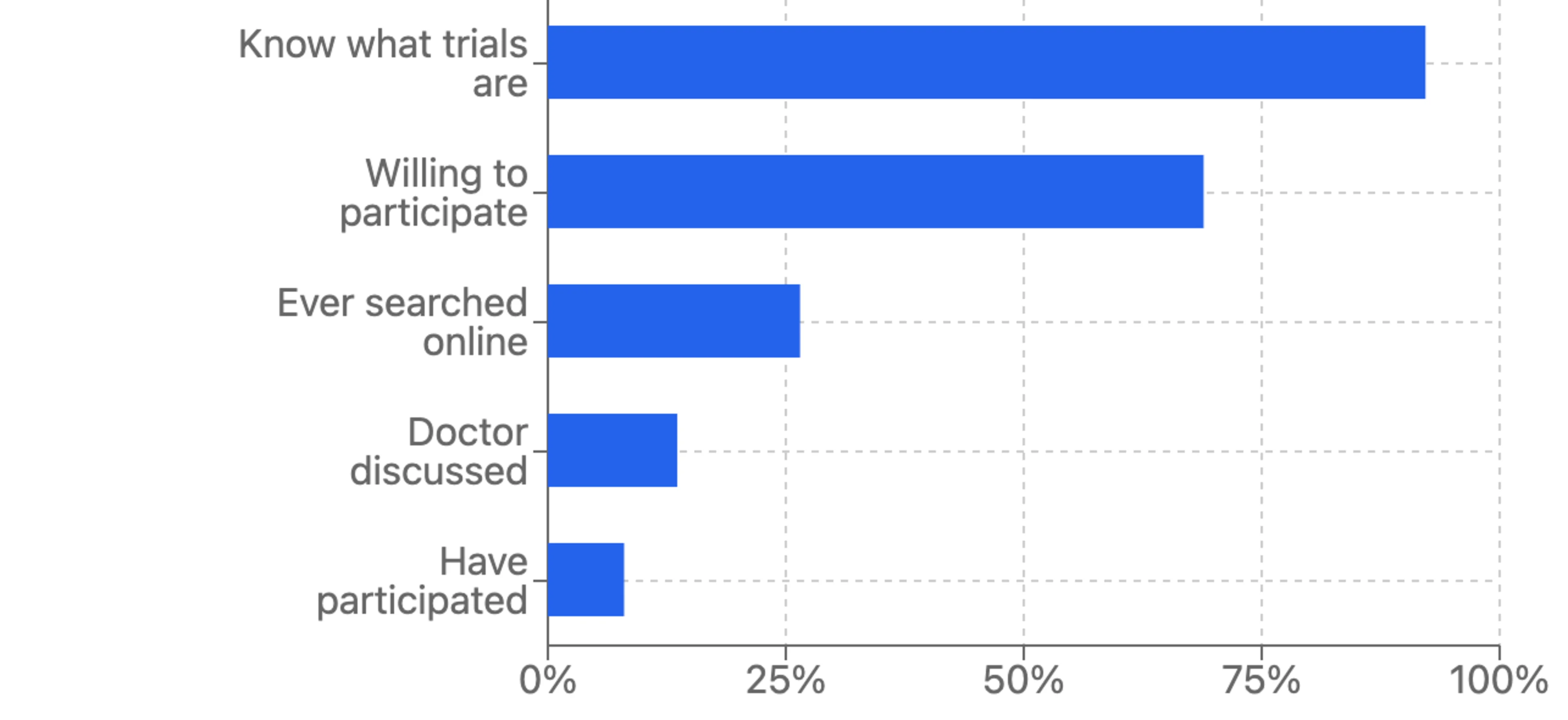
Significantly, while an overwhelming 92.2% of respondents know what clinical trials are, only 8% have ever participated in one. This disconnect between awareness and participation represents both a challenge and an opportunity for the clinical research community. This data reveals a major gap between knowledge and action. Most Americans understand the concept of clinical trials, but they’re not actively seeking them out or being referred to them by their physicians.
“We’re not seeing a lack of interest in advancing science. We’re seeing a breakdown in access, information, and trust,” explains David Franklin, co-founder of Unity Trials.
Over 60% of Americans aged 40 and above that were surveyed have never been invited to join a clinical trial. When asked if they would participate in a program evaluating a new drug or medical device, nearly 70% of respondents answered yes. Yet, nearly 50% said they wouldn’t know how to participate even if they wanted to.
Perhaps the most revealing finding is that only 14% of respondents report having a doctor discuss clinical trial participation with them, despite an overwhelming majority saying physicians are their primary source for medical information. This physician engagement gap represents a significant missed opportunity as healthcare providers are a trusted source of medical guidance for most Americans. Without physician referrals, many potential participants never learn about relevant clinical trial options.
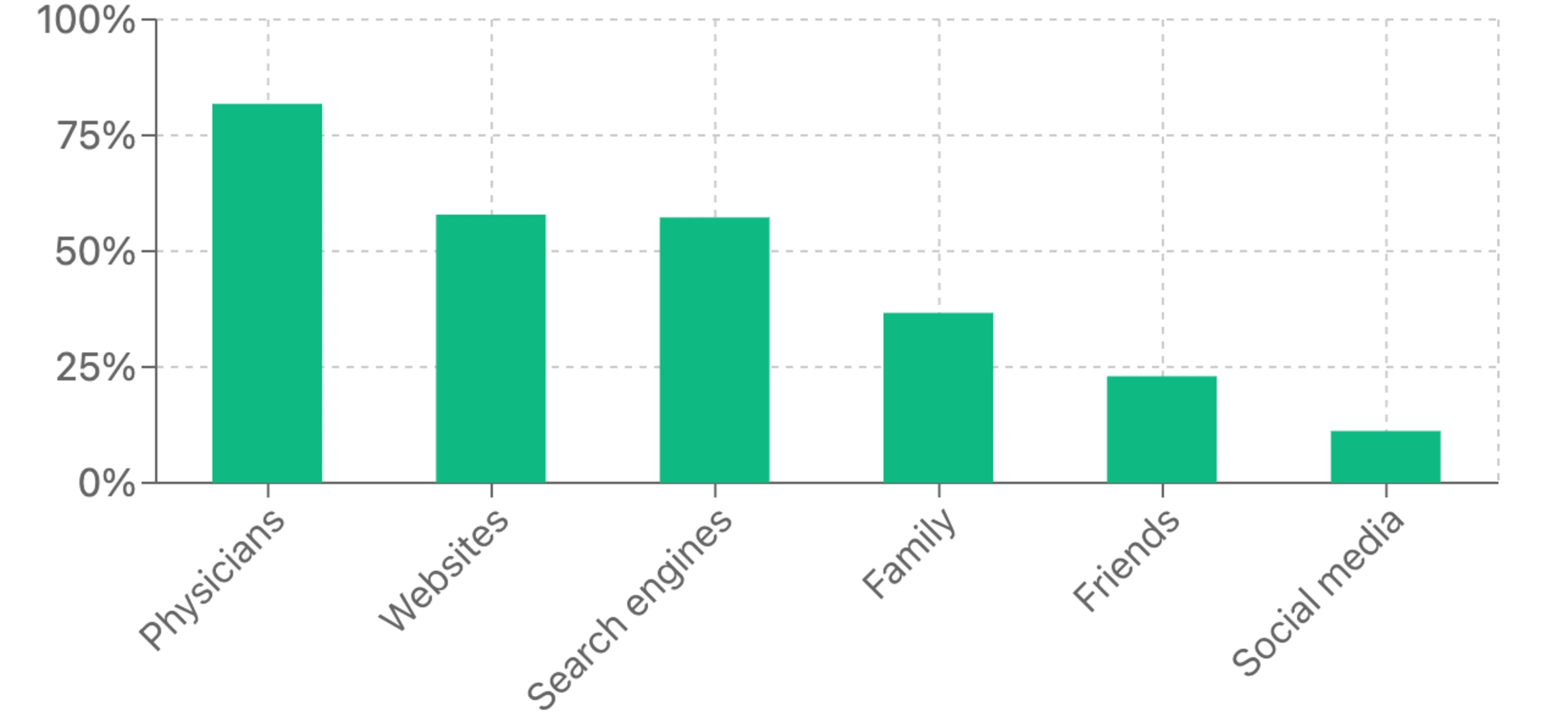
As clinical research evolves, the gap between awareness and participation represents both a challenge and an opportunity. By addressing key barriers—particularly physician engagement, trial formats, and geographic access—sponsors and clinicians can tap into the substantial interest in clinical trial participation. There isn’t an awareness problem or even a willingness problem. Instead, there’s a connection problem—interested participants aren’t being made aware of trials through trusted channels.
“Recruiting people over 50 should be the easy part,” Franklin continues. “Instead, it’s where the system is most broken. Fixing this and other issues are why we built UnityTrials.org.”
Unity Trials was started to help improve diversity among clinical trial participants in the United States. Clinical trials should reflect the population most likely to use the drug if it’s approved. So our concierge service works with individuals to match them with an individual trial aligned with their needs. We make finding and signing up for clinical trials easy, efficient, and inclusive.
This analysis is based on survey responses from 501 American adults across diverse age groups, genders, educational backgrounds, and geographic locations. The survey was conducted in 2025 with a margin of error of ±4.4% at the 95% confidence level.
Prefer a guided signup? Our team can help match you to an appropriate clinical trial.

Recent survey of Americans uncovered why people may or may not take part in medical research