
Clinical Trials Face a People Problem: New Data Reveals Americans Know About Clinical Trials, But Don’t Participate
See results of a March 2025 online survey of 501 Americans that probed awareness of clinical trials
We recently surveyed 501 Americans from across the country and uncovered surprising results related to an awareness-participation gap for clinical trials. In this article, we dive deeper into the reasons people may or may not want to take part in medical research.
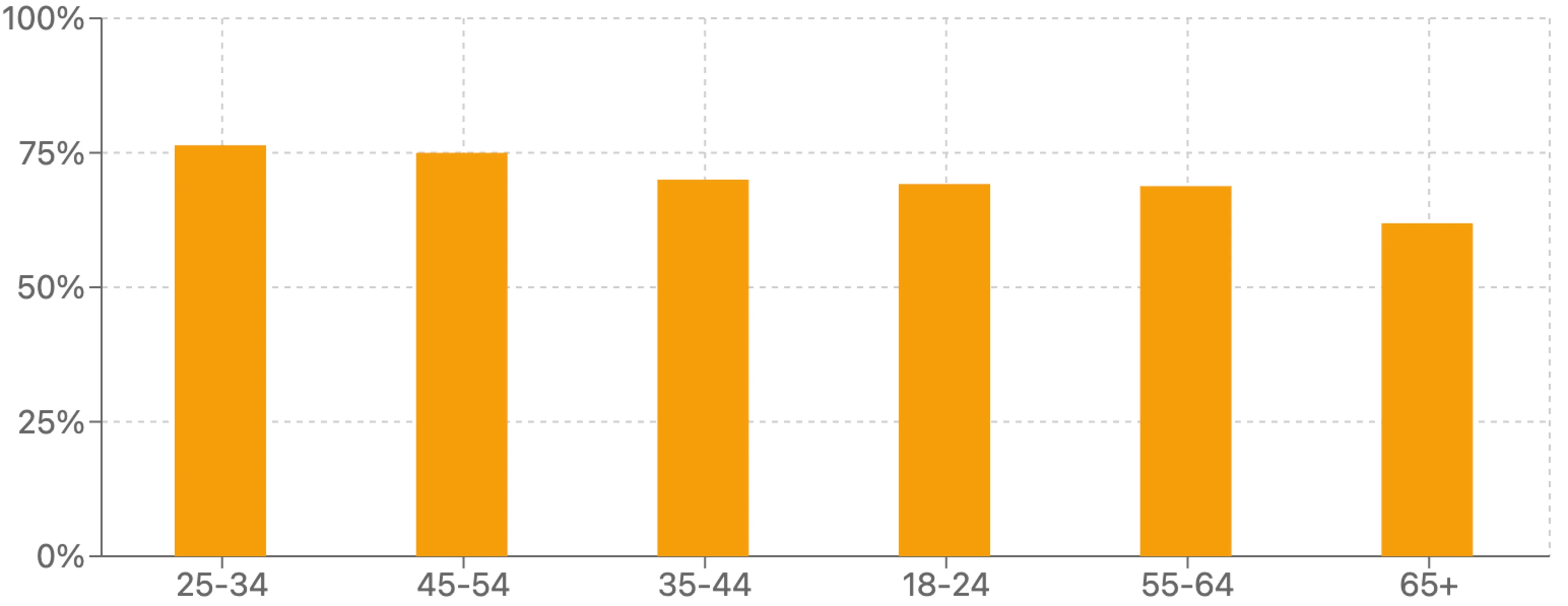
When we asked if they’d participate in a program evaluating a new drug or medical device, 69% of respondents said yes. Younger and middle-aged adults showed the highest willingness to participate with those aged 25-34 leading at 76%. Interest gradually declined with age, with those 65 and older showing the lowest interest at 62%.
Gender differences were also notable as men showed significantly higher willingness to participate (75%) compared to women (65%) in our survey. In 2021, academic researchers analyzed more than 280 clinical trials and found that women were underrepresented, accounting for just 1/3 of research participants.1 This is troubling because women and men are genetically and physiologically different. Sex‐dependent differences in renal clearance, body weight, and body fat percentage result in differences in drug disposition.2
“Diversity in trials isn’t just about ethnicity,” explains David Franklin, co-founder of Unity Trails. “It’s also about geography, education, and digital access—especially in rural and aging populations.”
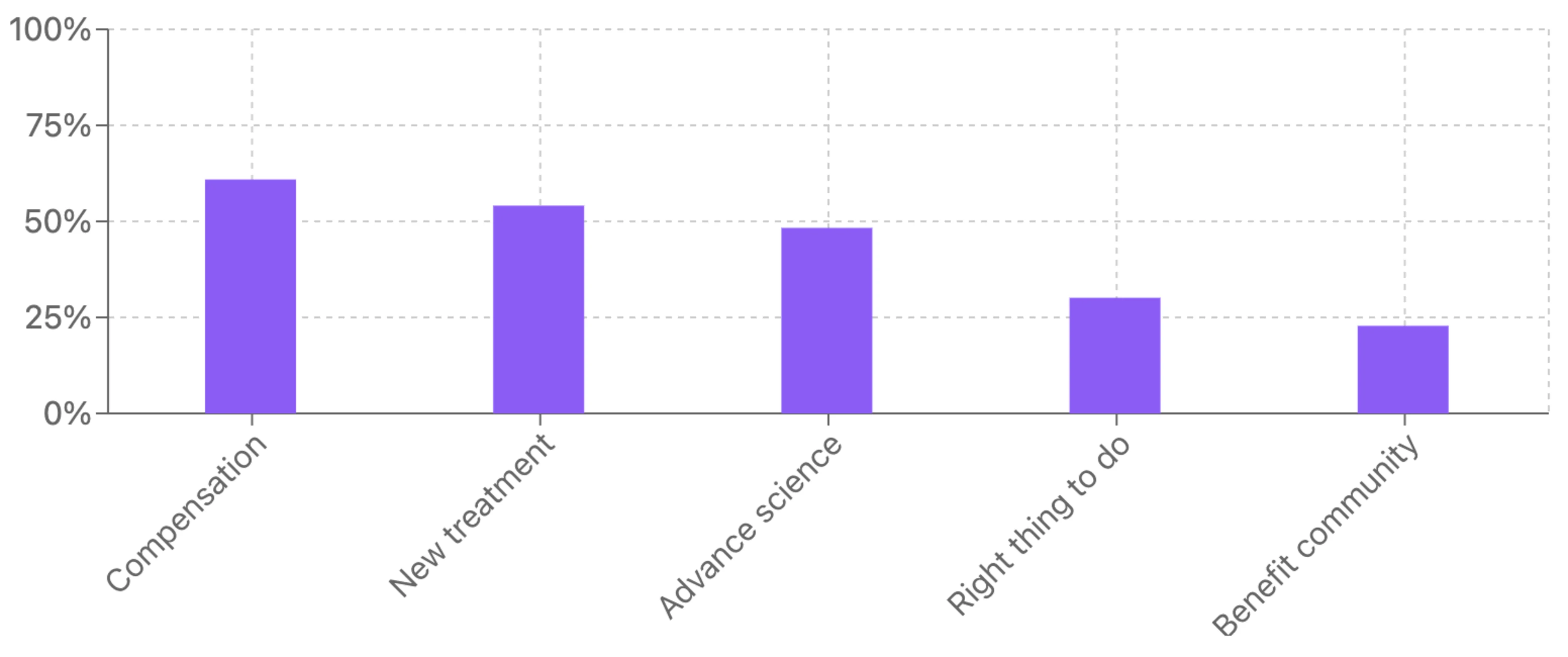
Top motivating factors for clinical trial participation balance financial, personal, and altruistic considerations. Unsurprisingly, financial incentives topped the list with 61% of respondents motivated by compensation. However, altruistic and personal health factors also play significant roles, with finding new treatments (54%) and advancing science (48%) ranking second and third, respectively.
Survey data suggests participants are motivated by a mix of these practical and idealistic factors. People want to help others and advance science, but they also value their time and recognize potential personal benefits from new treatments.
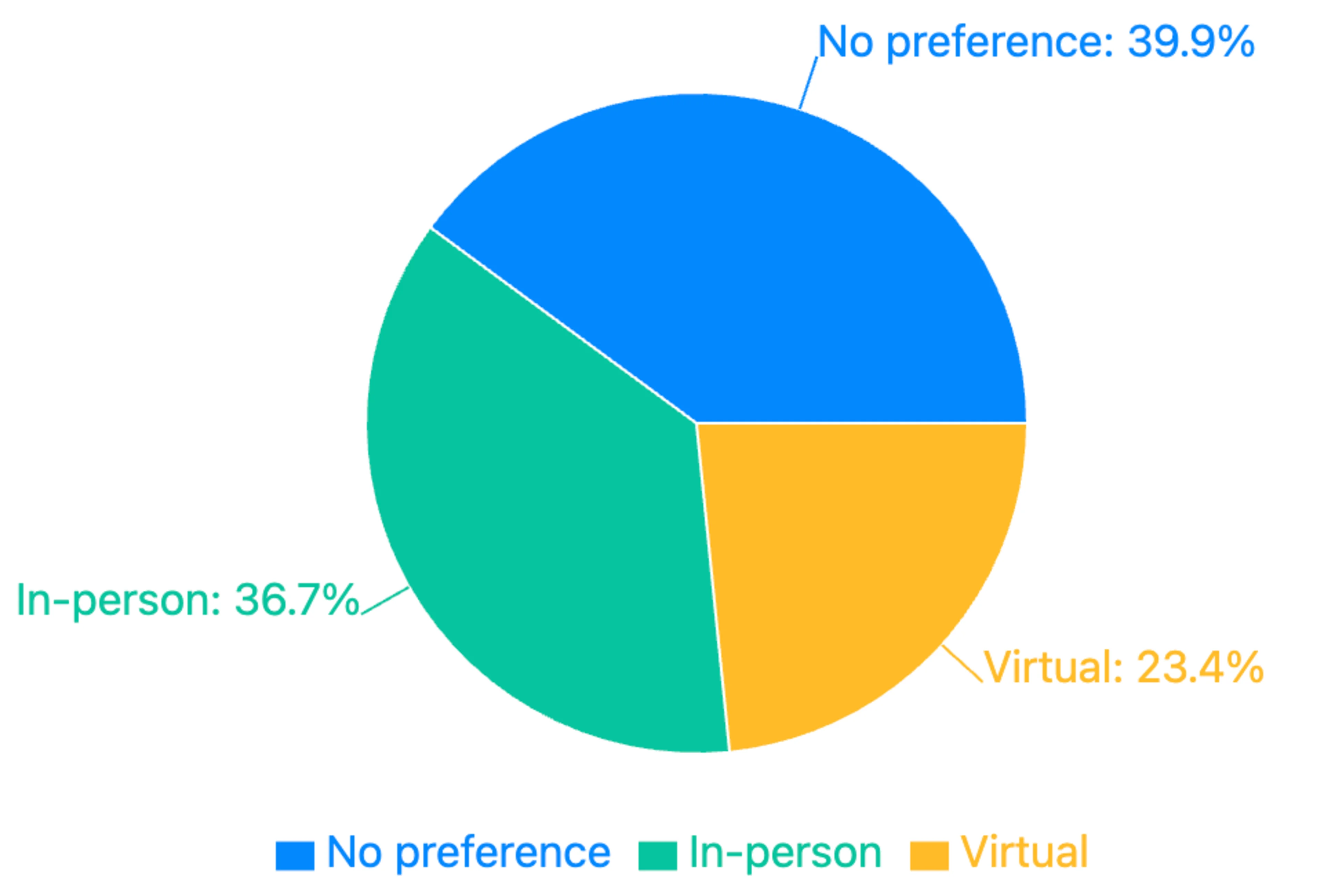
The COVID-19 pandemic accelerated the adoption of decentralized clinical trials, but our survey suggests Americans have mixed preferences regarding trial format. Nearly 40% of respondents expressed no preference. However, when preferences were expressed, in-person trials remained more popular than virtual ones.
The data revealed striking age-related differences as well. Younger adults showed much stronger preference for virtual trials than older adults, with those 18-24 (38.5%) being more than three times as likely to prefer virtual formats compared to those over 65 (12.3%).
Gender differences also emerged with women showing stronger preference for virtual trials compared to men. Proximity to trial sites remains a crucial factor in participation decisions. Only 22% of respondents would travel more than 30 minutes for a clinical trial.
Unity Trials is dedicated to connecting individuals with clinical trials, fostering greater participation and diversity to accelerate the development of innovative devices, diagnostics, and treatments. We can help you find a trial — whether it’s in-person, close to home, or focused on a condition close to your heart.
Unity Trials was started to help improve diversity among clinical trial participants in the United States. Clinical trials should reflect the population most likely to use the drug if it’s approved. So our concierge service works with individuals to match them with an individual trial aligned with their needs. We make finding and signing up for clinical trials easy, efficient, and inclusive.
This analysis is based on survey responses from 501 American adults across diverse age groups, genders, educational backgrounds, and geographic locations. The survey was conducted in 2025 with a margin of error of ±4.4% at the 95% confidence level.
1. Sex, Racial, and Ethnic Diversity in Clinical Trials. March 2023. Accessed April 25, 2024. www.ncbi.nlm.nih.gov/pmc/articles/PMC10264921 2. Spoletini I, Vitale C, et al. Sex Differences in Drug Effects: Interaction With Sex Hormones in Adult Life. Handb Exp Pharmacol. 2012;214:91‐105.
Prefer a guided signup? Our team can help match you to an appropriate clinical trial.

See results of a March 2025 online survey of 501 Americans that probed awareness of clinical trials